American consumers are being urged to check their medicine cabinets following a significant product recall over serious child safety concerns. Plantimex has issued a recall for its Mamisan Lidocaine Ointment, which was sold at major retailers Walmart and Target, both in-store and online.
Why the Ointment Poses a Deadly Threat
The urgent recall, announced by the U.S. Consumer Product Safety Commission (CPSC), centres on a critical packaging failure. The ointment was sold in containers without the legally required child-resistant packaging. This flaw means young children could easily open the jar, posing "a risk of serious injury or death from poisoning" if they were to swallow the lidocaine-containing product.
Under the long-standing Poison Prevention Packaging Act of 1970, medications and hazardous chemicals like this must be sold in packaging that is difficult for children under five to open. Regulators confirmed Plantimex's product was in violation of this crucial safety law.
Recall Details: What to Look For
The recall affects approximately 50,330 containers of the ointment, which was sold from April 2024 through October 2025 for around $10. The product is identified as follows:
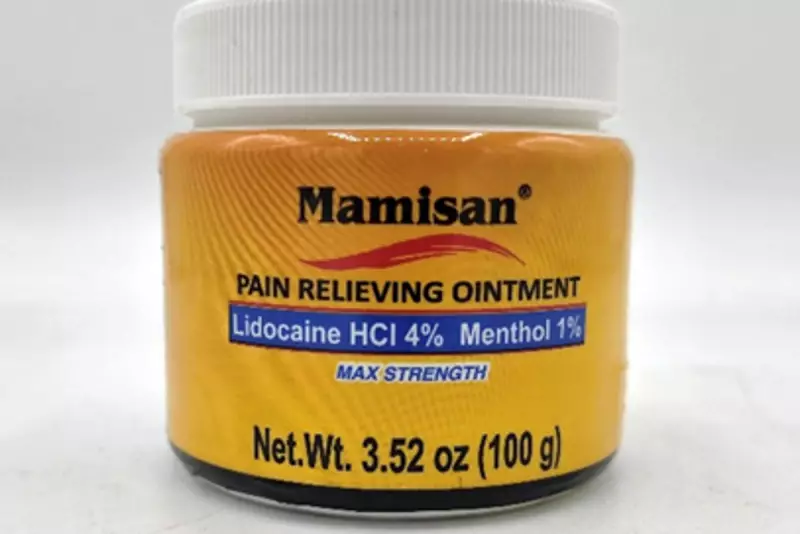
- Product: Mamisan Pain Relieving Topical Ointment.
- Packaging: A 3.52-ounce orange plastic jar with a white continuous thread lid.
- Markings: The Mamisan trademark is printed on both the lid and the container.
- UPC Code: Only jars with UPC code 860006498115 are included in this recall.
Immediate Steps for Consumers
As of Monday 22 December 2025, no injuries have been reported in connection with this defect. However, the CPSC and Plantimex advise immediate action for anyone who has purchased the product.
Consumers should immediately secure the recalled jars out of sight and reach of children. Do not continue using the ointment with the original lid.
Plantimex is providing a remedy. Customers can contact the company directly to receive a free replacement lid that meets child-resistant standards. Once the new, compliant lid is securely fitted, the ointment can be used as directed.
This recall serves as a stark reminder for all households to regularly review products in their homes, especially over-the-counter medications and topical treatments, ensuring they are stored safely and in their original, compliant packaging.





