A formal appeal has been launched against the NHS spending watchdog's controversial decision to withdraw a potentially life-saving cancer treatment from health services across England and Wales. The National Institute for Health and Care Excellence (Nice) recently recommended halting the continued rollout of Tecartus, a pioneering CAR T-cell therapy designed for patients with non-Hodgkin lymphoma.
Charities Challenge 'Backward Step' for Patient Care
Leading health charities Blood Cancer UK, Lymphoma Action and Anthony Nolan have jointly lodged the formal appeal, expressing profound concerns for patients diagnosed with relapsed or refractory mantle cell lymphoma. These individuals face severely limited alternative treatment options, making Tecartus their primary therapeutic hope.
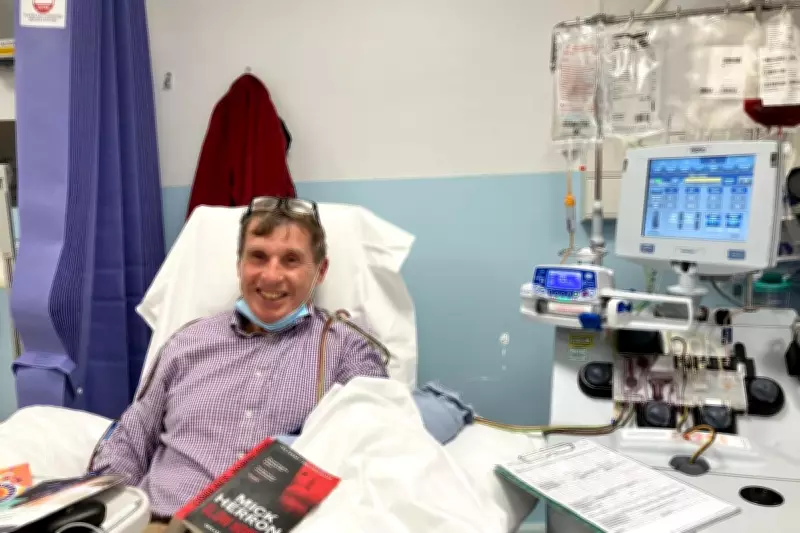
The treatment represents the only CAR T-cell therapy currently available for mantle cell lymphoma in the UK, a specific blood cancer affecting approximately 600 people annually. Paul Madley from Cardiff, who received his diagnosis in 2021, exemplifies the patients who could be impacted by this decision. His procedure to collect cells for treatment underscores the personal stakes involved.
Clinical Performance Concerns and Patient Safeguards
Nice's recommendation stems from observations that Tecartus did not perform as effectively in routine clinical practice as it had during controlled trial conditions. The watchdog has emphasised that patients already undergoing Tecartus treatment will be permitted to complete their therapeutic courses, ensuring continuity of care for those mid-treatment.
Furthermore, Nice confirmed that two alternative treatments for the same condition are currently undergoing evaluation, potentially offering future options. The organisation has stated it welcomes the appeal process as part of its transparent decision-making framework.
Wider Implications for NHS Cancer Care
Charities have described the potential withdrawal of Tecartus as representing a "significant backward step for NHS cancer care", particularly for patients with limited alternatives. The appeal process will now examine whether Nice's assessment adequately considered the unique circumstances of patients with relapsed or refractory mantle cell lymphoma.
This development highlights ongoing tensions between healthcare cost-effectiveness assessments and patient access to innovative treatments, especially for rare cancers where therapeutic options remain scarce. The outcome of this appeal could establish important precedents for how novel cancer therapies are evaluated and funded within the NHS framework.





