A Brazilian mother-of-one has been left in a critical condition after using an illegal counterfeit weight loss drug, suffering multiple seizures and requiring emergency surgery to help her breathe. The alarming case has prompted renewed warnings about the dangers of unregulated medications marketed online.
Severe Reaction to Unapproved Medication
Kellen Oliveira Bretas Antunes, a 42-year-old from Brazil, began using a drug called 'Lipoless' last month. The product is often marketed as a fat-burning capsule but is illegal to sell in Brazil as it lacks approval from the country's health agency, Anvisa. Just three days after starting the medication, Antunes experienced severe abdominal pain and body aches that forced her to seek urgent hospital treatment.
Hospitalization and Deteriorating Condition
Doctors initially diagnosed her with suspected drug intoxication, a reversible condition involving altered consciousness due to drug intake. She spent eight days in hospital before being discharged, but her health crisis was far from over. Three days later, she returned to medical care suffering from muscle weakness, dark urine, and the onset of seizures.
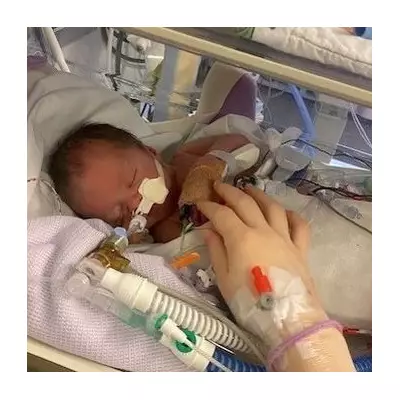
Since her readmission on December 28, Antunes has reportedly suffered sixteen seizures and required a tracheostomy procedure, where surgeons create an opening in the neck to facilitate breathing. Her daughter, Giulia Antunes, revealed in a social media post that her mother has now been diagnosed with Guillain-Barre Syndrome, a serious disorder where the immune system attacks the body's nerves, causing muscle tingling, numbness, and weakness.
Long Road to Recovery
'She is stable. There has been a significant improvement, but the process will be long, right?' her daughter told local media. 'Since she was diagnosed with Guillain-Barre Syndrome, it will take at least 12 months of treatment with physiotherapy, speech therapy and other specialists.'
Unclear Origins of Dangerous Product
While it remains unclear exactly how Antunes obtained the weight loss drug, her daughter has suggested it originated from Paraguay. Local media reports indicate that 'Lipoless' has been illegally imported into Brazil for use as a counterfeit weight loss product, sometimes referred to by the nickname 'Paraguayan pens'.
'My dad took the product to the hospital for tests, but they wouldn’t analyze it because it was medication from Paraguay,' Antunes' daughter explained to reporters.
Medical Community Issues Stern Warning
Flávia Coimbra, director of the Brazilian Society of Endocrinology and Metabolism, emphasized the extreme risks associated with using medications not approved by health authorities. 'There can be side-effects related to inadequate doses, hyperglycemia, and other adverse events such as contamination, serious infections, generalized infections, and worsening of pre-existing conditions,' she warned.
Regulatory Crackdown
In response to growing concerns, Brazil's health agency Anvisa recently banned two weight-loss drugs frequently marketed through social media platforms without proper registration. A spokesperson stated: 'The products were manufactured by unknown companies and are being advertised and sold through Instagram profiles without registration, notification, or listing with Anvisa. Because they are irregular products of unknown origin, there is no guarantee about their content or quality. Therefore, they should not be used under any circumstances.'
Global Concerns About Counterfeit Medications
This case echoes international warnings about the dangers of unregulated weight loss medications. The U.S. Food and Drug Administration has repeatedly cautioned approximately 13 million Americans currently taking GLP-1 medications for weight loss to avoid non-FDA-approved versions marketed online or through compounding pharmacies.
Maziar Mike Doustdar, CEO of Novo Nordisk (manufacturer of Ozempic and Wegovy), reinforced these concerns last week, noting that an estimated 1.5 million Americans are currently taking 'unsafe, knock-off versions of our products.'
Treatment Protocols and Medical Response
Medical professionals treat drug intoxication cases like Antunes' initial diagnosis through several methods:
- Pumping the stomach to remove substances
- Administering intravenous fluids
- Inducing vomiting when appropriate
For seizure management, doctors typically administer anti-seizure medications. When seizures cause prolonged breathing difficulties, tracheostomy procedures may become necessary to ensure adequate oxygen supply. Medical experts note that drug intoxication can sometimes cause airways to collapse, necessitating breathing tube insertion.
Ongoing Investigations and Wider Implications
Brazilian authorities are currently investigating Antunes' illness, while similar concerns persist internationally. According to FDA monitoring systems, at least ten deaths in the United States have been linked to compounded medications since 2023, though investigations to establish definitive causal links remain ongoing.
The FDA's latest warning in September noted 605 adverse events linked to compounded semaglutide and 545 reports for compounded tirzepatide as of July. These figures highlight the scale of potential risks associated with unregulated weight loss medications.
This distressing case serves as a stark reminder of the potentially life-threatening consequences of using unapproved medications, particularly those marketed through unofficial channels without proper regulatory oversight.