Nationwide Infant Botulism Outbreak Sparks Parental Warnings and Lawsuits
Families across the United States are sharing their traumatic experiences after their babies fell severely ill from a powdered infant formula now suspected of contamination with Clostridium botulinum spores. The situation has escalated into a significant national health scare, prompting a major voluntary recall and a growing number of legal actions against the manufacturer, New York-based ByHeart.
The Recall and Escalating Crisis
The unfolding crisis began on November 8, when ByHeart initiated a voluntary recall for two specific lots of its Whole Nutrition Infant Formula. This action came after the US Food and Drug Administration (FDA) alerted the company to an ongoing botulism investigation. Just three days later, on November 11, the recall was dramatically expanded to include all batches of the product.
The investigation gained traction after preliminary testing by the California Department of Public Health discovered Clostridium botulinum spores in an opened can of ByHeart formula that had been consumed by an infant who had become sick. Subsequently, ByHeart confirmed that independent, third-party laboratory tests had also detected the bacterium in some unopened cans, a finding the company reported to the FDA.
As of November 19, federal health officials report at least 31 cases of infant botulism across 15 states that are linked to the recalled formula. No fatalities have been reported, but the outbreak has triggered a wave of lawsuits from affected families.
Families Detail Harrowing Medical Ordeals
At least three new federal lawsuits, filed in Arizona, California, and Washington, detail strikingly similar medical emergencies. In each case, previously healthy infants suddenly developed profound weakness, feeding difficulties, and life-threatening neurological symptoms after consuming the formula.
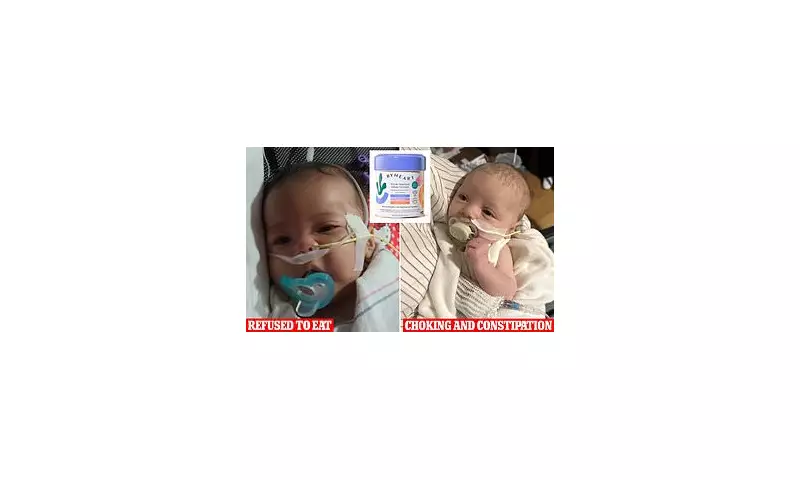
One lawsuit comes from Stephen and Yurany Dexter in Arizona. Their daughter, identified in court documents as E.D., was born on July 5, 2025, and initially thrived. By August 21, her parents noticed troubling signs: stomach discomfort, gas, and a steadily decreasing appetite. The suit claims that within a week, she refused to eat entirely, lost the ability to suck and swallow, and could no longer hold her head up.
Doctors at Phoenix Children's Hospital initially suspected muscular dystrophy due to the rarity of infant botulism. As her condition deteriorated, E.D. was given BabyBIG antitoxin, the only specific treatment for botulism. She was eventually discharged with a feeding tube and, her parents allege, continues to struggle with digestive and strength issues, alongside separation anxiety.
In California, a lawsuit centres on infant A.B., born September 29, 2025. After being fed ByHeart formula exclusively, he was hospitalised in late October. Medical records cited in the suit describe a near-total loss of strength, an inability to latch, and extreme lethargy. A stool sample later confirmed botulism type A. Although he has since improved, his parents say he continues to struggle with constipation and slow feeding, stating the ordeal 'shattered the trust' they placed in the brand.
Another suit from Washington state involves the infant daughter of Madison and Tyler Wescott. After showing symptoms including difficulty feeding, choking, and extreme fatigue in early November, she was hospitalised and treated for confirmed botulism, remaining in care until November 19.
Legal Repercussions and Company Response
Lead attorney Bill Marler of Marler Clark, The Food Safety Law Firm, told the Daily Mail he has now been retained by 'over a dozen families' whose children are part of the outbreak and expects more cases to be identified. He is also reviewing earlier 2025 infant botulism cases where ByHeart formula was consumed.
In a statement to the Daily Mail, ByHeart called the situation 'heartbreaking' and stated that the safety of babies is its highest priority. The company acknowledged that its independent lab identified Clostridium botulinum in some samples of its formula.
ByHeart stated, 'We are working in cooperation with the FDA, and we are investigating every facet of our process. All findings will be shared transparently... Until now, this bacterium was not among the pathogens routinely tested for across the industry.' The company has set up 24/7 support channels for concerned families.
Understanding Infant Botulism
Infant botulism is a rare but serious condition that occurs when spores of the Clostridium botulinum bacterium colonise an infant's intestines and produce a potent neurotoxin. Unlike foodborne botulism in adults, the food itself does not contain the pre-formed toxin; it contains the spores that then produce the toxin inside the baby's body.
Symptoms to watch for include:
- Constipation
- Poor feeding and a weak suck
- Drooping eyelids
- Lethargy and weakened cry
- Loss of head control and general muscle weakness
The primary treatment is an antitoxin called Botulism Immune Globulin Intravenous (BIG-IV or BabyBIG). While the death rate is very low with modern medical care, recovery can be a lengthy process requiring extensive hospitalisation and therapy. Health authorities strongly advise never giving honey to infants under 12 months, as it is a known source of the spores.






