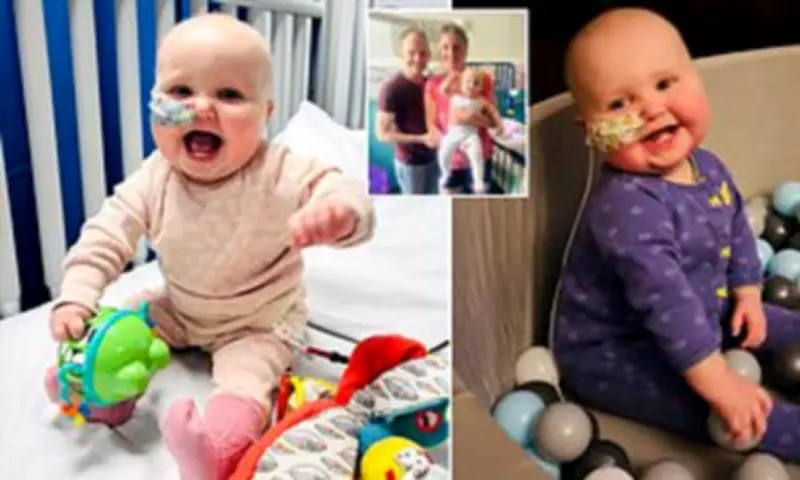
Poole Infant's Desperate Race for £500,000 Lifesaving Cancer Treatment
The family of a one-year-old girl battling a deadly form of blood cancer have received the devastating news that she has mere weeks left to live unless they can raise half a million pounds for a life-saving drug available only in the United States. Melody Aggett, from Poole in Dorset, was diagnosed with acute myeloid leukaemia (AML) when she was just four months old, plunging her family into a medical nightmare that has now reached its most critical stage.
A Harrowing Medical Journey
Since her diagnosis in April 2025, baby Melody has endured an arduous treatment regimen that has included multiple rounds of intensive chemotherapy, regular blood and platelet transfusions, and extended periods in intensive care as medical teams fought to control the aggressive disease. Her parents, Rachel Elizabeth and Kevin Aggett, have watched their daughter suffer through every possible side effect of the powerful medications, with each treatment bringing new challenges and complications.
In what doctors described as the final treatment option available within Britain's healthcare system, Melody underwent a bone marrow transplant at Great Ormond Street Hospital in August 2025. Tragically, this last-ditch effort failed when she developed acute graft-versus-host disease, a severe complication where donated cells attack the recipient's body, followed by a relapse of her cancer in October.
The Devastating Conversation
"We were taken into a room and told there is nothing else they can do," recalled Mr Aggett, 40, describing the moment medical professionals delivered the crushing news. "Our first thought was, 'Well, are you sure?' We were told Melody needed palliative care and that the hospital would put that in place. We were also advised to go home and spend the last few weeks that we would have with Melody together."
Rather than accepting this prognosis, the determined parents began researching alternative options online, where they discovered Revuforj - an FDA-approved oral medication that has shown success in treating Melody's specific type of leukaemia in American clinical trials. The drug works by slowing the growth of cancer cells and has demonstrated promising remission rates, though it remains unavailable through NHS funding pathways.
Regulatory Breakthrough but Financial Mountain
Melody's oncology team at University Hospital Southampton submitted an emergency specialist request to the Medicines & Healthcare products Regulatory Agency, which granted permission for the drug to be legally imported from the United States for her treatment. This regulatory approval represents a significant breakthrough, but it comes with an astronomical financial burden that the family cannot shoulder alone.
The treatment carries a staggering price tag of £20,500 per month, with Melody expected to require the medication for up to two years - bringing the total cost to approximately £500,000. "It's a long-term drug, so she will need to have it more than likely for two years," explained Ms Elizabeth, 34. "This is why we need to raise such a significant amount of money."
The Urgent Fundraising Campaign
The family has established a GoFundMe page in a desperate attempt to raise the necessary funds, though their current total of nearly £60,000 covers only three months of treatment. Medical urgency adds tremendous pressure to their efforts, as doctors have indicated they would have started the treatment immediately if it were available.
"We asked our consultant last week if they had this drug in front of them right now, when would they give it to Melody," said Ms Elizabeth. "And they said, 'I would have started it yesterday for her.' There may come a point very soon that Melody gets too poorly that we can't use it. Now, her leukaemia levels are okay - but that could change at any point, so this is very urgent."
Broader Implications for UK Healthcare
Beyond their personal struggle, Melody's parents hope their daughter's case might pave the way for other British children facing similar diagnoses. "We want to help Melody and to save Melody," Ms Elizabeth emphasised, "but we're also hoping that by us using this drug and Melody using this drug, that will help other children in the future, and it will help other people to be able to access it going forward."
The family's situation highlights the difficult intersection of cutting-edge medical treatments, regulatory approvals, and healthcare funding limitations within the UK system. As they race against time to secure the financial resources needed for their daughter's survival, they maintain hope that both Melody and future patients might benefit from access to innovative treatments that currently exist beyond NHS provision.





