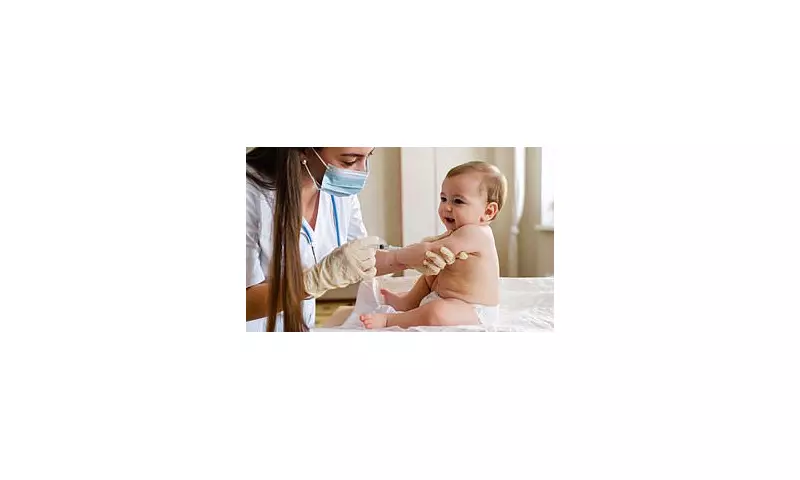
In a significant policy shift, the US Centers for Disease Control and Prevention (CDC) has formally ended its long-standing recommendation that all newborns receive the hepatitis B vaccine within 24 hours of birth. The decision, approved by acting director Jim O'Neill, moves towards a model of individual-based decision-making for infants born to parents without the virus.
A New Framework for Vaccination
The agency's new guidance states that while the shot can still be administered at birth, the first dose for low-risk infants may now also be given when babies are at least two months old. This change follows a recommendation made two weeks prior by the CDC's Advisory Committee on Immunization Practices (ACIP). However, the CDC maintains that children born to parents who have hepatitis B should still receive the vaccination immediately after birth.
Notably, Mr O'Neill did not approve a second ACIP proposal. This would have seen parents and healthcare providers consider blood tests to check newborns' antibody levels against hepatitis B to determine if additional doses of the three-shot regimen were needed. The acting director stated the agency was still reviewing this recommendation.
Controversy and Medical Backlash
The policy change has sparked considerable debate within the medical community. The ACIP itself has been a focus of controversy since its membership was hand-picked by vaccine-sceptic Health and Human Services Secretary, Robert F Kennedy Junior. Major medical associations have expressed strong opposition to delaying the birth dose.
Dr Susan Kressly, president of the American Academy of Pediatrics, issued a stark warning. "Pediatricians are already reporting more parents declining to give their children this critical dose," she said. "It is deeply disappointing to see the continued dismissal of expertise to inform recommendations that have broad implications on the health of America's children."
Despite the change, major health insurers, including Blue Cross Blue Shield Association and AHIP, have confirmed they will continue to cover all vaccines recommended at the start of 2025 through 2026, including the hepatitis B birth dose.
Understanding the Risks and the Vaccine
Hepatitis B is a viral infection that attacks the liver and can cause lifelong scarring, liver failure, and cancer. It is incurable. Estimates suggest up to 2.4 million US adults are infected, with about 640,000 having a chronic, long-term infection. The virus spreads through exposure to infected blood or bodily fluids and can survive on surfaces for a week.
The risks are particularly severe for infants. Up to 90 percent of babies infected with hepatitis B develop the chronic condition, which can lead to severe complications and death later in life. The World Health Organization states the three-dose vaccine prevents infection in more than 95 percent of healthy infants, children, and young adults, and it has a strong safety profile from rigorous trials.
In defending the new policy, Jim O'Neill said it "reflects ACIP's rigorous review of the available evidence" and restores "the balance of informed consent to parents whose newborns face little risk of contracting hepatitis B."
The US first approved the hepatitis B vaccine in 1982 and recommended it for infants from 1991. In 2005, it tightened guidance to insist on a dose within 24 hours of birth. ACIP members debating the change noted that while countries like the UK and Denmark delay the first dose until two months, the US has a more diverse, larger population without a universal healthcare system, making direct comparisons difficult.
Current estimates indicate about 20,000 infants are born to mothers with hepatitis B annually, yet only about 10 of those children become infected each year. Approximately 91.4 percent of US children have received three doses of the vaccine by age 24 months.